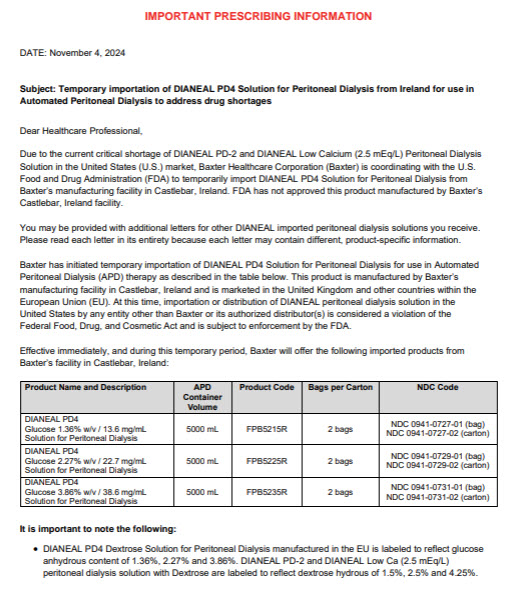
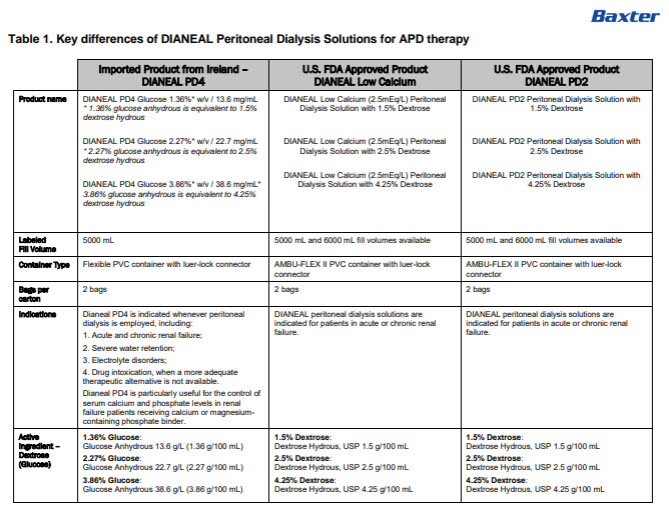
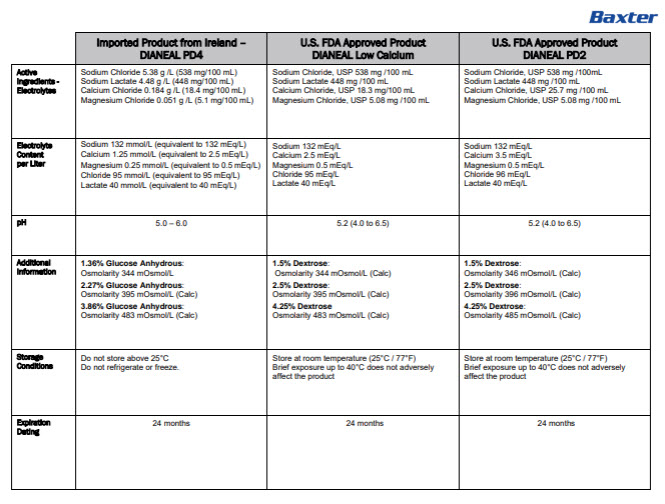
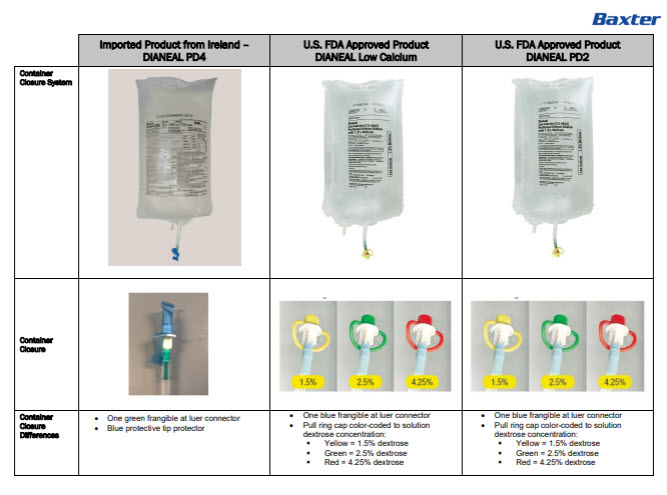
Dianeal Low Calcium Peritoneal Dialysis Solution: Package Insert / Prescribing Info
Package insert / product label
Generic name: sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose
Dosage form: injection, solution
Drug class: Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Mar 17, 2025.
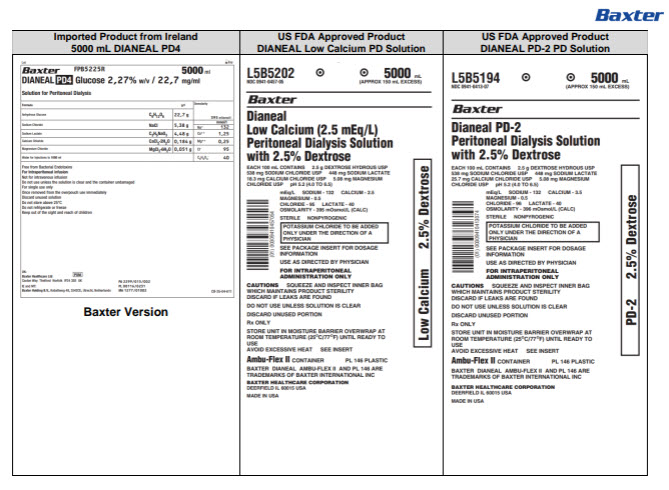
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
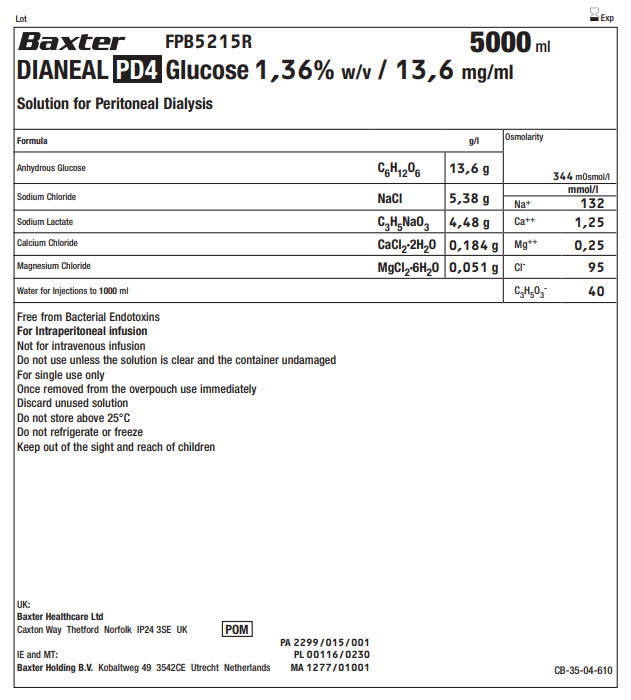
Lot Exp
BaxterLogo FPB5215R 5000 ml
DIANEAL PD4 Glucose 1,36% w/v / 13,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
Osmolarity |
344 mOsmol/l |
|
|
Anhydrous Glucose |
C 6H 12O 6 |
13,6 g |
||
|
Sodium Chloride |
NaCl |
5,38 g |
Na + |
mmol/l |
|
132 |
||||
|
Sodium Lactate |
C 3H 5NaO 3 |
4,48 g |
Ca ++ |
1,25 |
|
Calcium Chloride |
CaCl 2·2H 2O |
0,184 g |
Mg ++ |
0,25 |
|
Magnesium Chloride |
MgCl 2·6H 2O |
0,051 g |
Cl - |
95 |
|
Water for Injections to 1000ml |
C 3H 5O 3- |
40 |
||
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of children
UK:
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE UK
POM symbol
IE and MT:
Baxter Holding B.V.Kobaltweg 49 354CE Utrecht Netherlands
PA 2299/015/001
PL 00116/0230
MA 1277/01001
CB-35-04-610
FPB5215R 5000 ml x 2
Dianeal PD4 Glucose 1.36% w/v / 13,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
|
Anhydrous Glucose |
13,6 g |
|
Sodium Chloride |
5,38 g |
|
Sodium Lactate |
4,48 g |
|
Calcium Chloride |
0,184 g |
|
Magnesium Chloride |
0,051 g |
|
Water for Injections to 1000 ml |
|
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.
UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 354CE Utrecht, Netherlands
PA 2299/015/001 PL 00116/0230 MA 1277/01001
Lot:
Single bag with
Luer connector
Do not store above 25°C.
Do not refrigerate or
freeze.
88-46-13-198
POM symbol
Barcode
(01)50085412574375(17)201200(10)88B88B88B8
Dianeal PD4 Glucose
1.36% w/v / 13,6mg/ml
Lot:
FPB5215R
5000 ml x 2
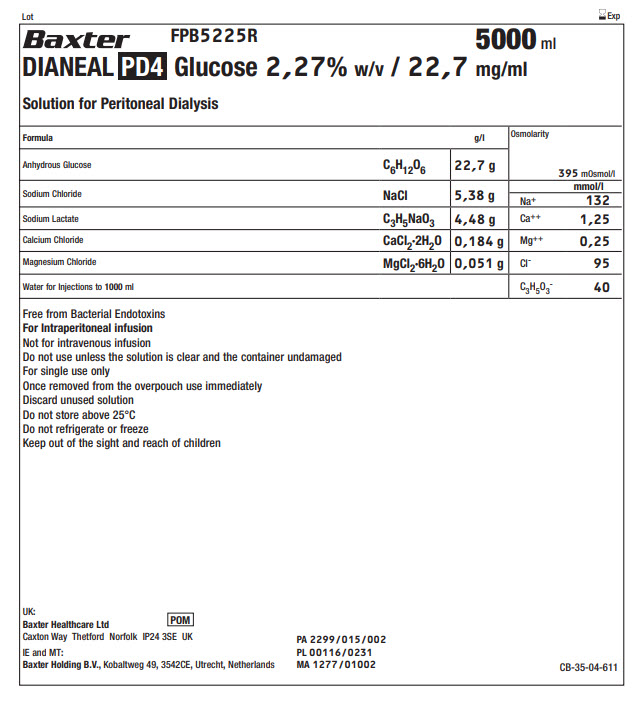
Lot Exp
BaxterLogo FPB5225R 5000 ml
DIANEAL PD4 Glucose 2,27% w/v / 22,7 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
Osmolarity |
395 mOsmol/l |
|
|
Anhydrous Glucose |
C 6H 12O 6 |
22,7 g |
||
|
Sodium Chloride |
NaCl |
5,38 g |
Na + |
mmol/l |
|
132 |
||||
|
Sodium Lactate |
C 3H 5NaO 3 |
4,48 g |
Ca ++ |
1,25 |
|
Calcium Chloride |
CaCl 2·2H 2O |
0,184 g |
Mg ++ |
0,25 |
|
Magnesium Chloride |
MgCl 2·6H 2O |
0,051 g |
Cl - |
95 |
|
Water for Injections to 1000ml |
C 3H 5O 3- |
40 |
||
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of children
UK:
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE UK
POM symbol
IE and MT:
Baxter Holding B.V.,Kobaltweg 49, 354CE, Utrecht, Netherlands
PA 2299/015/002
PL 00116/0231
MA 1277/01002
CB-35-04-611
FPB5225R 5000 ml x 2
Dianeal PD4 Glucose 2.27% w/v / 22,7 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
|
Anhydrous Glucose |
22,7 g |
|
Sodium Chloride |
5,38 g |
|
Sodium Lactate |
4,48 g |
|
Calcium Chloride |
0,184 g |
|
Magnesium Chloride |
0,051 g |
|
Water for Injections to 1000 ml |
|
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.
UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 354CE Utrecht, Netherlands
PA 2299/015/002 PL 00116/0231 MA 1277/01002
Lot:
Single bag with
Luer connector
Do not store above 25°C.
Do not refrigerate or
freeze.
88-46-13-199
POM symbol
Barcode
(01)50085412574382(17)201200(10)88B88B88B8
Dianeal PD4 Glucose
2.27% w/v / 22,7mg/ml
Lot:
FPB5225R
5000 ml x 2
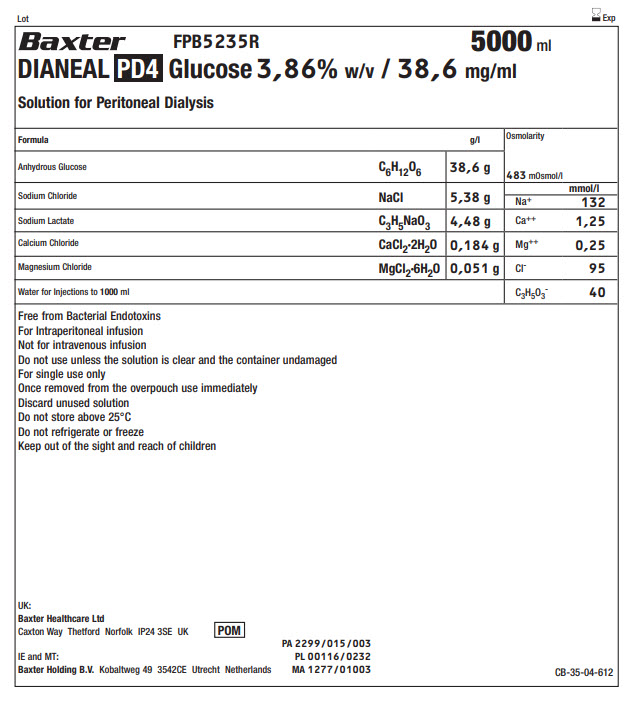
Lot Exp
BaxterLogo FPB5235R 5000 ml
DIANEAL PD4 Glucose 3,86% w/v / 38,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
Osmolarity |
395 mOsmol/l |
|
|
Anhydrous Glucose |
C 6H 12O 6 |
38,6 g |
||
|
Sodium Chloride |
NaCl |
5,38 g |
Na + |
mmol/l |
|
132 |
||||
|
Sodium Lactate |
C 3H 5NaO 3 |
4,48 g |
Ca ++ |
1,25 |
|
Calcium Chloride |
CaCl 2·2H 2O |
0,184 g |
Mg ++ |
0,25 |
|
Magnesium Chloride |
MgCl 2·6H 2O |
0,051 g |
Cl - |
95 |
|
Water for Injections to 1000ml |
C 3H 5O 3- |
40 |
||
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of children
UK:
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE UK
POM symbol
IE and MT:
Baxter Holding B.V.Kobaltweg 49 354CE Utrecht Netherlands
PA 2299/015/003
PL 00116/0232
MA 1277/01003
CB-35-04-612
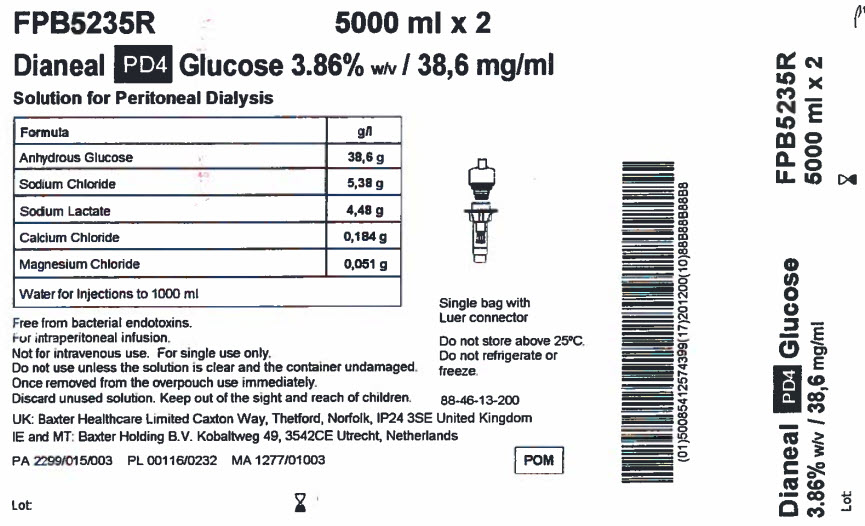
FPB5235R 5000 ml x 2
Dianeal PD4 Glucose 3.86% w/v / 38,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
|
Anhydrous Glucose |
38,6 g |
|
Sodium Chloride |
5,38 g |
|
Sodium Lactate |
4,48 g |
|
Calcium Chloride |
0,184 g |
|
Magnesium Chloride |
0,051 g |
|
Water for Injections to 1000 ml |
|
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.
UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 354CE Utrecht, Netherlands
PA 2299/015/003 PL 00116/0232 MA 1277/01003
Lot:
Single bag with
Luer connector
Do not store above 25°C.
Do not refrigerate or
freeze.
88-46-13-200
POM symbol
Barcode
(01)50085412574399(17)201200(10)88B88B88B8
Dianeal PD4 Glucose
3.86% w/v / 38,6mg/ml
Lot:
FPB5235R
5000 ml x 2
Lot Exp
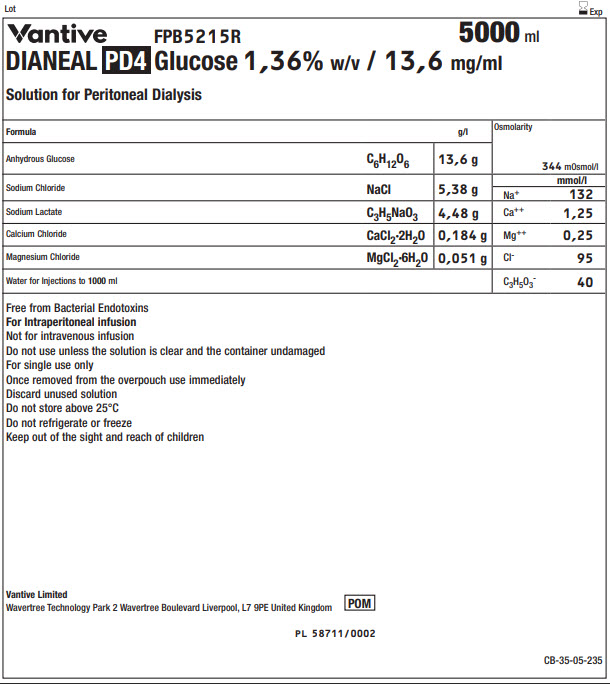
Vantive Logo FPB5215R 5000 ml
DIANEAL PD4 Glucose 1,36% w/v / 13,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
Osmolarity |
344 mOsmol/l |
|
|
Anhydrous Glucose |
C 6H 12O 6 |
13,6 g |
||
|
Sodium Chloride |
NaCl |
5,38 g |
Na + |
mmol/l |
|
132 |
||||
|
Sodium Lactate |
C 3H 5NaO 3 |
4,48 g |
Ca ++ |
1,25 |
|
Calcium Chloride |
CaCl 2·2H 2O |
0,184 g |
Mg ++ |
0,25 |
|
Magnesium Chloride |
MgCl 2·6H 2O |
0,051 g |
Cl - |
95 |
|
Water for Injections to 1000ml |
C 3H 5O 3- |
40 |
||
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of children
Vantive Limited
Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
POM symbol
PL 58711/0002
CB-35-05-235
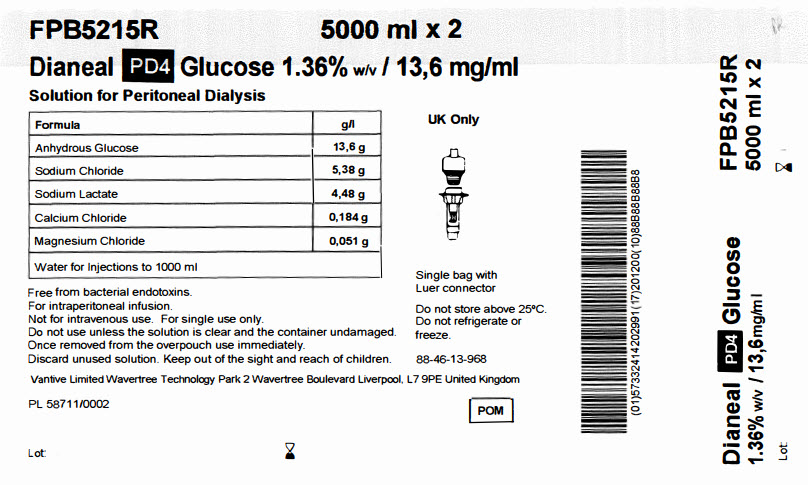
FPB5215R 5000 ml x 2
Dianeal PD4 Glucose 1.36% w/v / 13,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
|
Anhydrous Glucose |
13,6 g |
|
Sodium Chloride |
5,38 g |
|
Sodium Lactate |
4,48 g |
|
Calcium Chloride |
0,184 g |
|
Magnesium Chloride |
0,051 g |
|
Water for Injections to 1000 ml |
|
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.
Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
PL 58711/0002
Lot:
Single bag with
Luer connector
Do not store above 25°C.
Do not refrigerate or
freeze.
88-46-13-968
POM symbol
Barcode
(01)57332414202991(17)201200(10)88B88B88B8
Dianeal PD4 Glucose
1.36% w/v / 13,6mg/ml
Lot:
FPB5215R
5000 ml x 2
Lot Exp
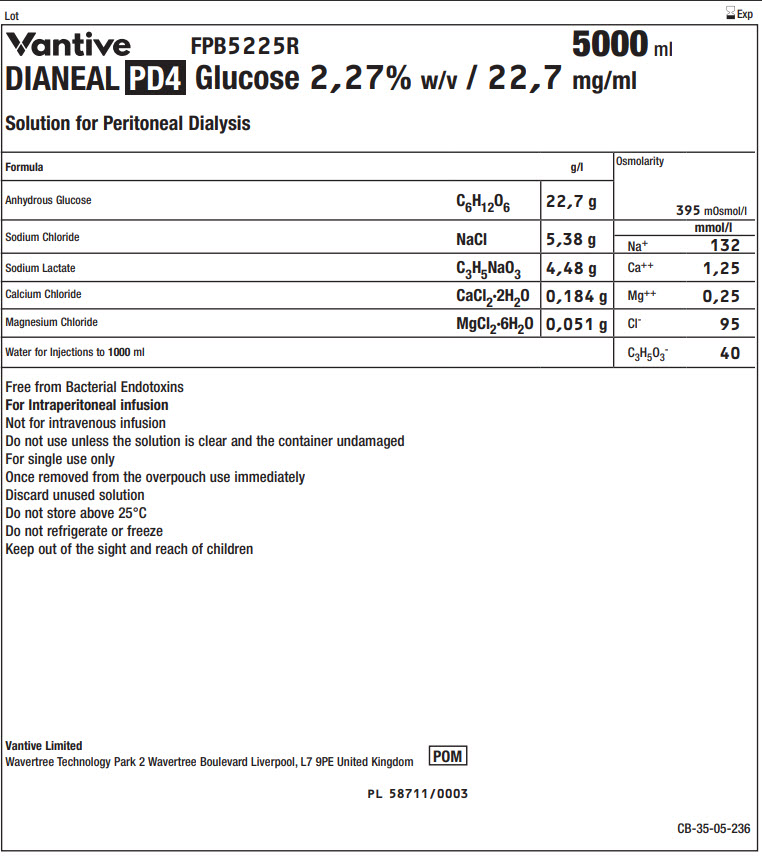
Vantive Logo FPB5225R 5000 ml
DIANEAL PD4 Glucose 2,27% w/v / 22,7 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
Osmolarity |
395 mOsmol/l |
|
|
Anhydrous Glucose |
C 6H 12O 6 |
22,7 g |
||
|
Sodium Chloride |
NaCl |
5,38 g |
Na + |
mmol/l |
|
132 |
||||
|
Sodium Lactate |
C 3H 5NaO 3 |
4,48 g |
Ca ++ |
1,25 |
|
Calcium Chloride |
CaCl 2·2H 2O |
0,184 g |
Mg ++ |
0,25 |
|
Magnesium Chloride |
MgCl 2·6H 2O |
0,051 g |
Cl - |
95 |
|
Water for Injections to 1000ml |
C 3H 5O 3- |
40 |
||
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of children
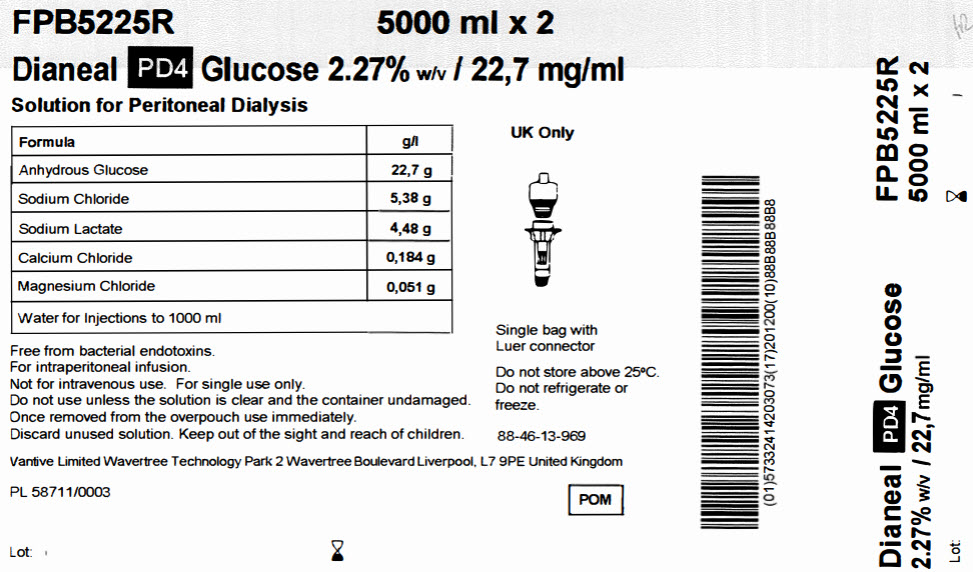
Vantive Limited
Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
POM symbol
PL 58711/0003
CB-35-05-236
FPB5225R 5000 ml x 2
Dianeal PD4 Glucose 2.27% w/v / 22,7 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
|
Anhydrous Glucose |
22,7 g |
|
Sodium Chloride |
5,38 g |
|
Sodium Lactate |
4,48 g |
|
Calcium Chloride |
0,184 g |
|
Magnesium Chloride |
0,051 g |
|
Water for Injections to 1000 ml |
|
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.
Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
PL 58744/0003
Lot:
Single bag with
Luer connector
Do not store above 25°C.
Do not refrigerate or
freeze.
88-46-13-969
POM symbol
Barcode
(01)57332414203073(17)201200(10)88B88B88B8
Dianeal PD4 Glucose
2.27% w/v / 22,7mg/ml
Lot:
FPB5225R
5000 ml x 2
Lot Exp
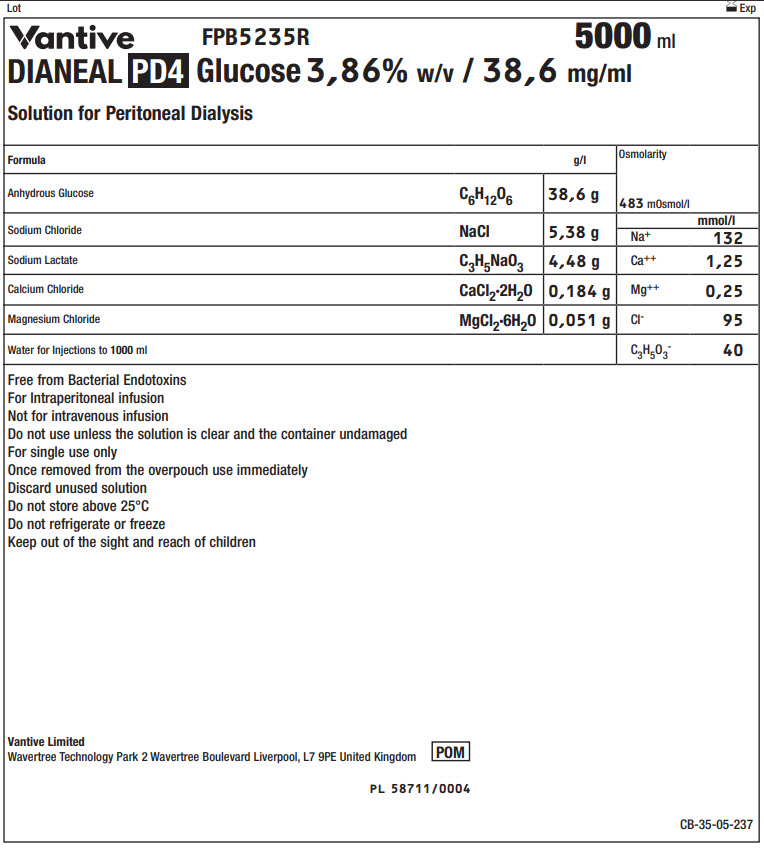
Vantive Logo FPB5235R 5000 ml
DIANEAL PD4 Glucose 3,86% w/v / 38,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
Osmolarity |
395 mOsmol/l |
|
|
Anhydrous Glucose |
C 6H 12O 6 |
38,6 g |
||
|
Sodium Chloride |
NaCl |
5,38 g |
Na + |
mmol/l |
|
132 |
||||
|
Sodium Lactate |
C 3H 5NaO 3 |
4,48 g |
Ca ++ |
1,25 |
|
Calcium Chloride |
CaCl 2·2H 2O |
0,184 g |
Mg ++ |
0,25 |
|
Magnesium Chloride |
MgCl 2·6H 2O |
0,051 g |
Cl - |
95 |
|
Water for Injections to 1000ml |
C 3H 5O 3- |
40 |
||
Free from Bacterial Endotoxins
For Intraperitoneal infusion
Not for intravenous infusion
Do not use unless the solution is clear and the container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Do not store above 25°C
Do not refrigerate or freeze
Keep out of the sight and reach of children
Vantive Limited
Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
POM symbol
PL 58711/0004
CB-35-05-237
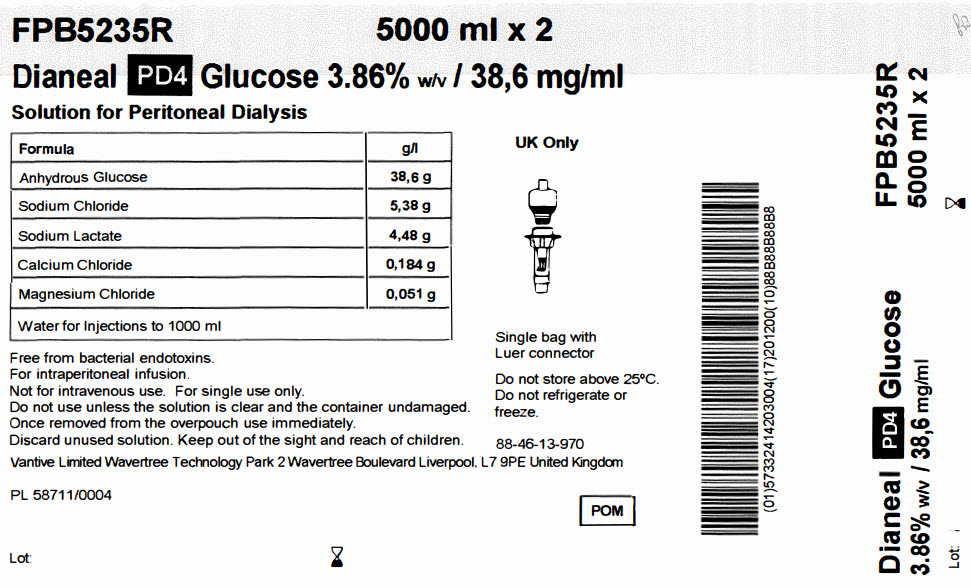
FPB5235R 5000 ml x 2
Dianeal PD4 Glucose 3.86% w/v / 38,6 mg/ml
Solution for Peritoneal Dialysis
|
Formula |
g/l |
|
Anhydrous Glucose |
38,6 g |
|
Sodium Chloride |
5,38 g |
|
Sodium Lactate |
4,48 g |
|
Calcium Chloride |
0,184 g |
|
Magnesium Chloride |
0,051 g |
|
Water for Injections to 1000 ml |
|
Free from bacterial endotoxins.
For intraperitoneal infusion.
Not for intravenous use. For single use only.
Do not use unless the solution is clear and the container undamaged.
Once removed from the overpouch use immediately.
Discard unused solution. Keep out of the sight and reach of children.
Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
PL 58711/0004
Lot:
Single bag with
Luer connector
Do not store above 25°C.
Do not refrigerate or
freeze.
88-46-13-970
POM symbol
Barcode
(01)57332414203004(17)201200(10)88B88B88B8
Dianeal PD4 Glucose
3.86% w/v / 38,6mg/ml
Lot:
FPB5235R
5000 ml x 2
| DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Vantive US Healthcare LLC (119181963) |
More about lvp solution
Patient resources
Professional resources
Other brands
Lactated Ringers Injection, Normosol-R, Extraneal, Delflex, ... +3 more